Our representatives are available to schedule your appointment Monday through Friday from 9am to 5pm.
For a Northwell ambulance, call
(833) 259-2367.
Holding a loved one’s hand is a simple, quiet gesture that can offer comfort, particularly during difficult times. But when Keith Thomas, 45, actually felt his sister Michelle’s hand for the first time in three years, it felt more like a “burst of energy,” he said during a recent session in my lab at the Feinstein Institutes for Medical Research.
A diving accident had paralyzed Keith from the chest down, leading to Michelle becoming his primary caregiver. Watching the raw emotions Keith and Michelle felt in this moment, I know Keith felt more than a burst of energy — he felt hope.
This was Michelle’s first visit to our lab, but we’ve been working with Keith every Tuesday and Thursday for more than a year as part of a first-of-its-kind clinical trial testing new technology that is restoring lasting movement and sensations in parts of his arm and hand. He is the first person to benefit from what is called a "double neural bypass," which reconnects the brain, body and spinal cord electronically in people living with paralysis. The technology uses a combination of brain implants, artificial intelligence (AI), and non-invasive electrodes placed over muscles and on the back of the neck to deliver thought-driven stimulation to restore movement and sensation, potentially for good.
On top of new sensations in his hand and wrist, Keith can now move his arms at will and has double the arm strength he had at the start of the trial. Even more impressive: He can perform some basic tasks, like drinking out of a cup. While we take for granted the motor skills involved in that motion, Keith cherishes each small movement of his fingers, hand and arm that makes it possible. And these improvements aren’t limited to the lab — he continues to feel and move even when he isn’t connected to the system.
Our goal is to use this technology one day to give people, like Keith, the ability to live fuller, more independent lives.
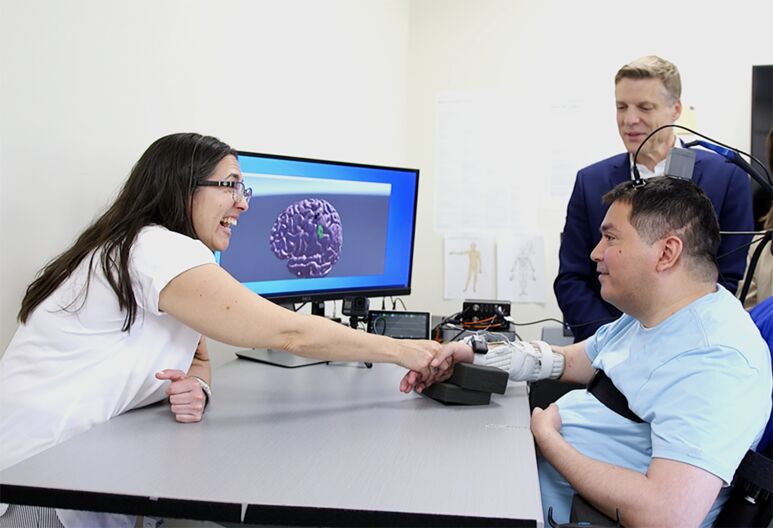
For the first time in years, Keith Thomas (front right) feels the touch of his sister's hand thanks to the double neural bypass, which included a 15-hour surgery at North Shore University Hospital, and months of work with researchers at the Feinstein Institutes for Medical Research, led by Chad Bouton (back right).
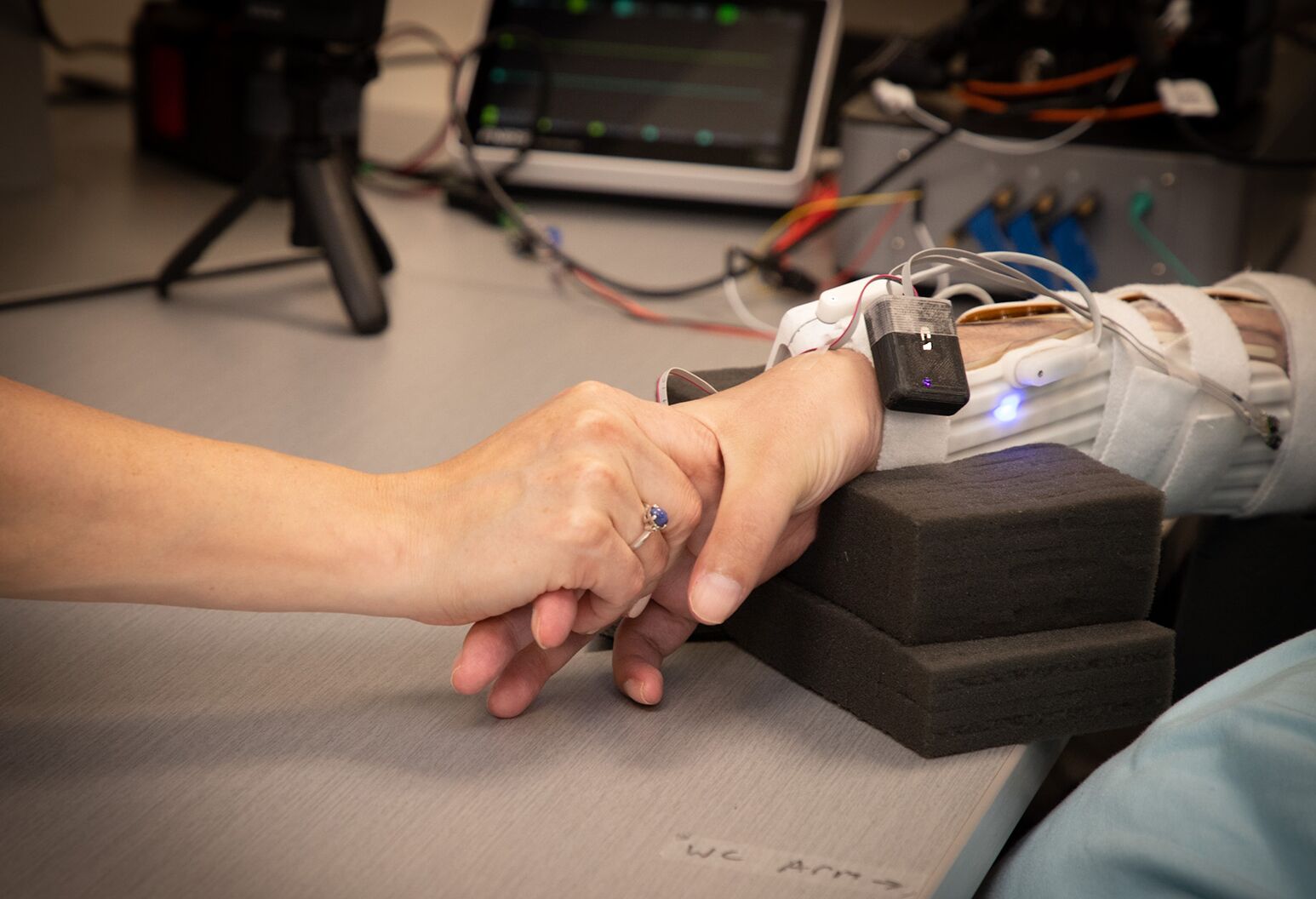
Patches on Keith Thomas' neck and arm play an integral role in the double neural bypass, which is helping to restore communication to and from his brain and allowing him to feel sensation in parts of his hand and arm.
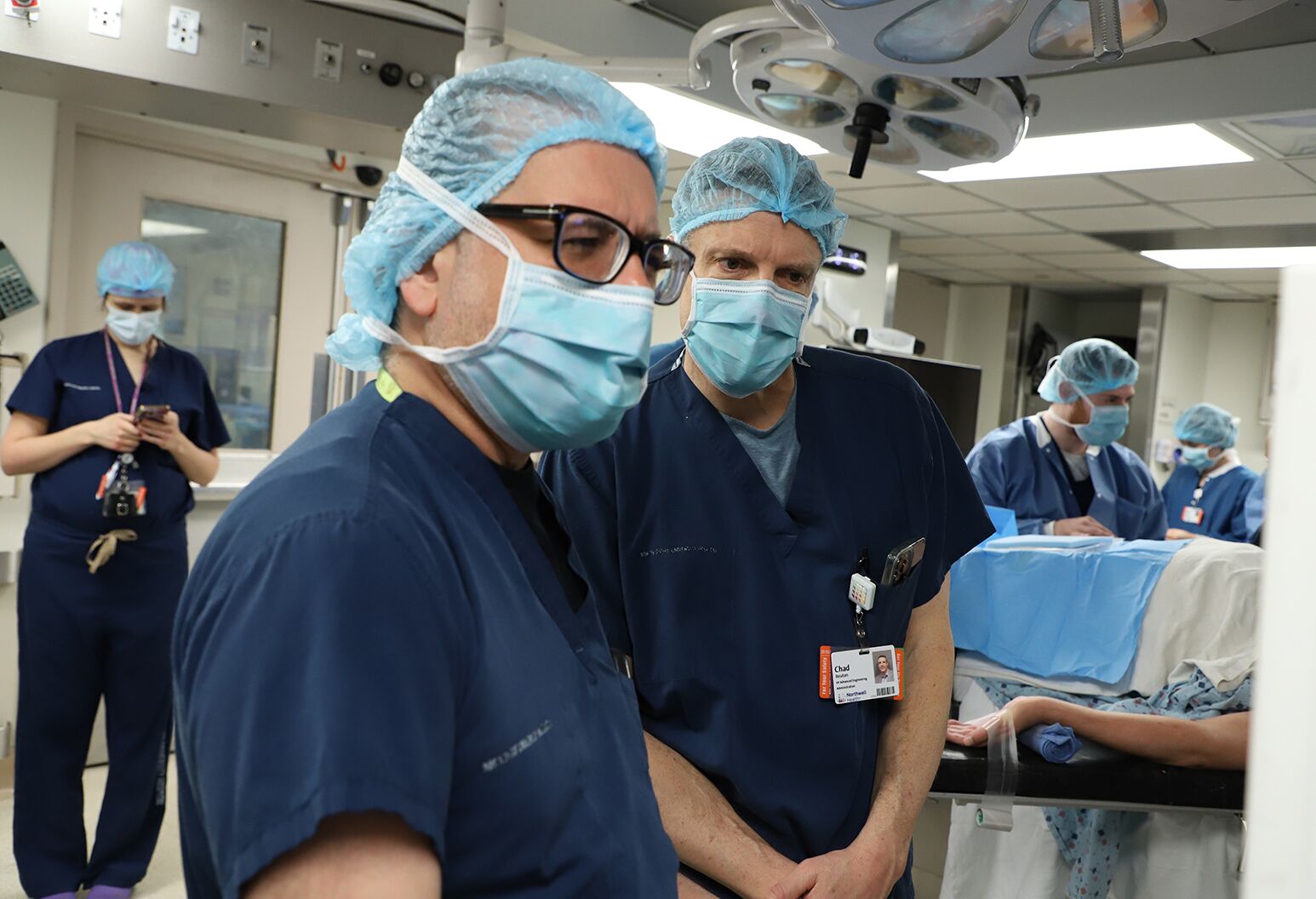
Ashesh Mehta, MD, (left) and Prof. Chad Bouton (right), prepare to insert a critical portion of the double neural bypass, restoring not only lasting movement in a man with quadriplegia, but the sense of touch in his arm and hand.
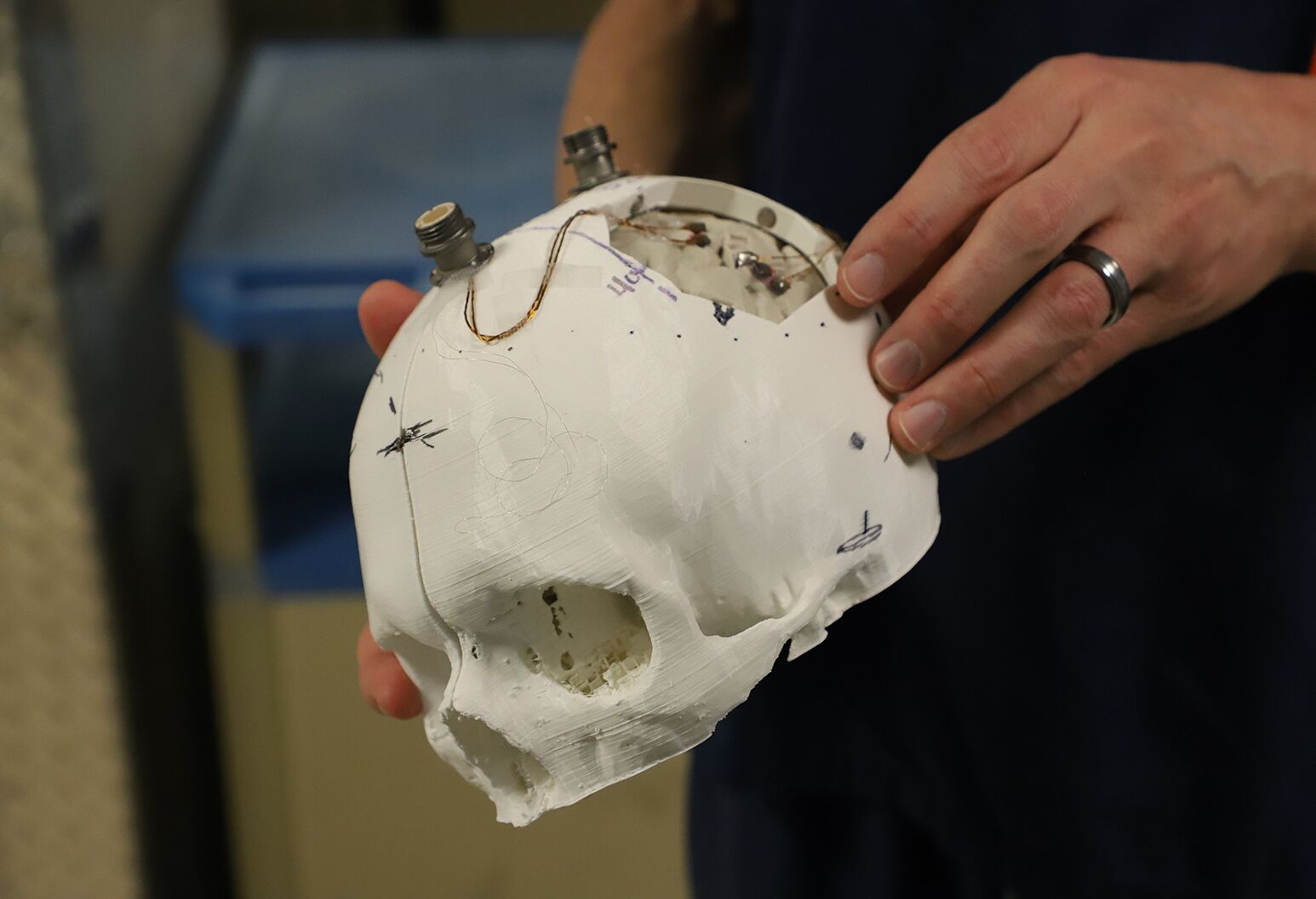
Feinstein Institutes’ bioelectronic medicine researchers hold a 3D model of the skull and brain of a man with quadriplegia, used to help identify where to place five tiny computer chips in the brain.

Keith Thomas, who lives with paralysis, poses with the research team at Northwell Health’s Feinstein Institutes for Medical Research, including (from left) Erona Ibroci, MS, Aniket Jangam, MS, Santosh Chandrasekaran, PhD, Zeev Elias, MS, Christina Maffei and Chad Bouton.

The implants are inserted into Keith's head and connected directly to microchips implanted on the surface of his brain.

Feinstein experts, Aniket Jangam, MS (left), and, Zeev Elias, MS (right) work with Keith Thomas (front) to fine-tune the implants and calibrate his fine motor skills.
On July 18, 2020, Keith broke his neck while diving into a pool resulting in a complete C4-C5 level spinal cord injury. As a result of his accident, he became quadriplegic with no movement or sensation below his chest. He was hospitalized for eight months in the midst of the Covid-19 pandemic’s second wave — a time when visitors still were not permitted in hospitals. The feeling of being trapped cannot begin to describe how Keith felt during that time. I recall him saying that he didn’t know if he was going to survive it — or if he wanted to.
No longer able to care for himself, the Manhattanite had to leave behind his Madison Avenue apartment and fast-paced lifestyle and move in with his sister’s family on Long Island.
Keith had joined the 5.4 million Americans living with paralysis — the two leading causes are stroke and spinal cord injury. Approximately half of those living with spinal cord injury lose movement in all four limbs, like Keith, making tasks like getting out of bed, getting dressed or brushing your teeth impossible. There is currently no cure for chronic paralysis. Worldwide, there are approximately 100 million people living with some form of movement impairment or paralysis.
Then there’s the sense of touch, which plays a fundamental role in our every waking moment. Most people take it for granted, but a day doesn’t go by when Keith isn’t reminded of his profound loss of sensation. One day he’s with his friends by the pool, the next he’s in a hospital bed unable to move or feel.
After discharge from the hospital, Keith started rehab in the STARS program at Northwell Health to learn how to live with his new limitations — a process he did not fully embrace. Recognizing that his patient was struggling to find purpose, his doctor Adam Stein, MD, connected me with Keith in hopes that our work in movement restoration could help.
Spinal cord injuries such as Keith’s are one of the causes of movement disabilities, along with stroke, multiple sclerosis, Parkinson’s disease, ALS, and other neurodegenerative conditions.
When I was in graduate school, I sustained a traumatic brain injury leading to expressive aphasia (inability to speak). I went through extensive physical, occupational, and speech therapy, but was lucky enough to regain what I had once lost. Since then, I have dedicated my life to developing new technologies to treat people who are living with paralysis and have not been as fortunate in their recoveries.
The spinal cord is comprised of millions of neurons, which relay and process messages between the brain and the body, making your every move, breath and sensation possible. When someone has a spinal cord injury, those messages are interrupted because the vertebrae that normally protect the cord fracture and shift, damaging the spinal cord and, depending on the location and severity, can lead to differing levels of paralysis.
In my research, I have focused on restoring movement and sensation to the hands, which studies and surveys indicate is a top desire for people living with quadriplegia. In 2010, I proposed the idea of a (single) "neural bypass" that would act as a one-way electronic bridge between the brain and paralyzed muscles to restore movement in paralyzed humans. My colleagues and I spent several years developing the first neural bypass system that used a single brain implant, machine learning, and novel stimulation electrodes to restore volitional movement. In 2014, our trial participant became the first paralyzed person to regain movement using a brain implant linked electronically to his muscles. Our work was published in Nature (2016), paving the way for future studies and new developments in the field of brain-computer interfaces — also called neuroprosthetics — a rapidly growing field opening many new possibilities in the field of medicine. Our trial participant was able to use his hand to feed and groom himself again. It was an incredible moment. But something was still missing — not only did they have to be in a lab, hooked up to computers to achieve this new ability, he mentioned that he still couldn’t feel any of the objects he was now able to use.
A few years later, through the Feinstein’s Institute for Bioelectronic Medicine, we began to pinpoint locations in the brain related to sensation in the hand, specifically for the fingertips.
This brings us back to Keith.
Building on what we learned, we developed non-invasive muscle and spinal cord stimulation technology to form the first double neural bypass system.
Our team, including Santosh Chandrasekaran, PhD, Erona Ibroci, MS, Zeev Elias, MS, Aniket Jangam, MS, Matthew Glasser, MD, PhD, (WUSTL), Adam Stein, MD, Stephan Bickel, MD, Christina Maffei, Doug Griffin, and many others, spent months working with Keith and performing functional MRI’s to carefully map his brain to locate the motor area, responsible for movement, and the sensory area, responsible for the sense of touch. With that information, surgeons implanted five microchips, each smaller than the tip of a pinky finger and made of etched silicon coated with a thin layer of metal — enabling them to record electrical activity. In total we had 224 electrodes spread across those five microchips, designed to enable us to listen in on individual neurons in those areas and essentially intercept and reroute those signals to the part of the body for which they were intended.
The 15-hour surgery took place on March 8, 2023 at NSUH and was led by Ashesh Mehta, MD, PhD, director of Northwell's Laboratory for Human Brain Mapping, and Netanel Ben-Shalom, MD, neurosurgeon at Northwell Lenox Hill Hospital. Before implanting the electrodes, we had to confirm that the positions we determined in our pre-surgical mapping were accurate. Keith was awakened, mid-surgery, to give real-time verbal feedback — the first test of this portion of the double neural bypass.
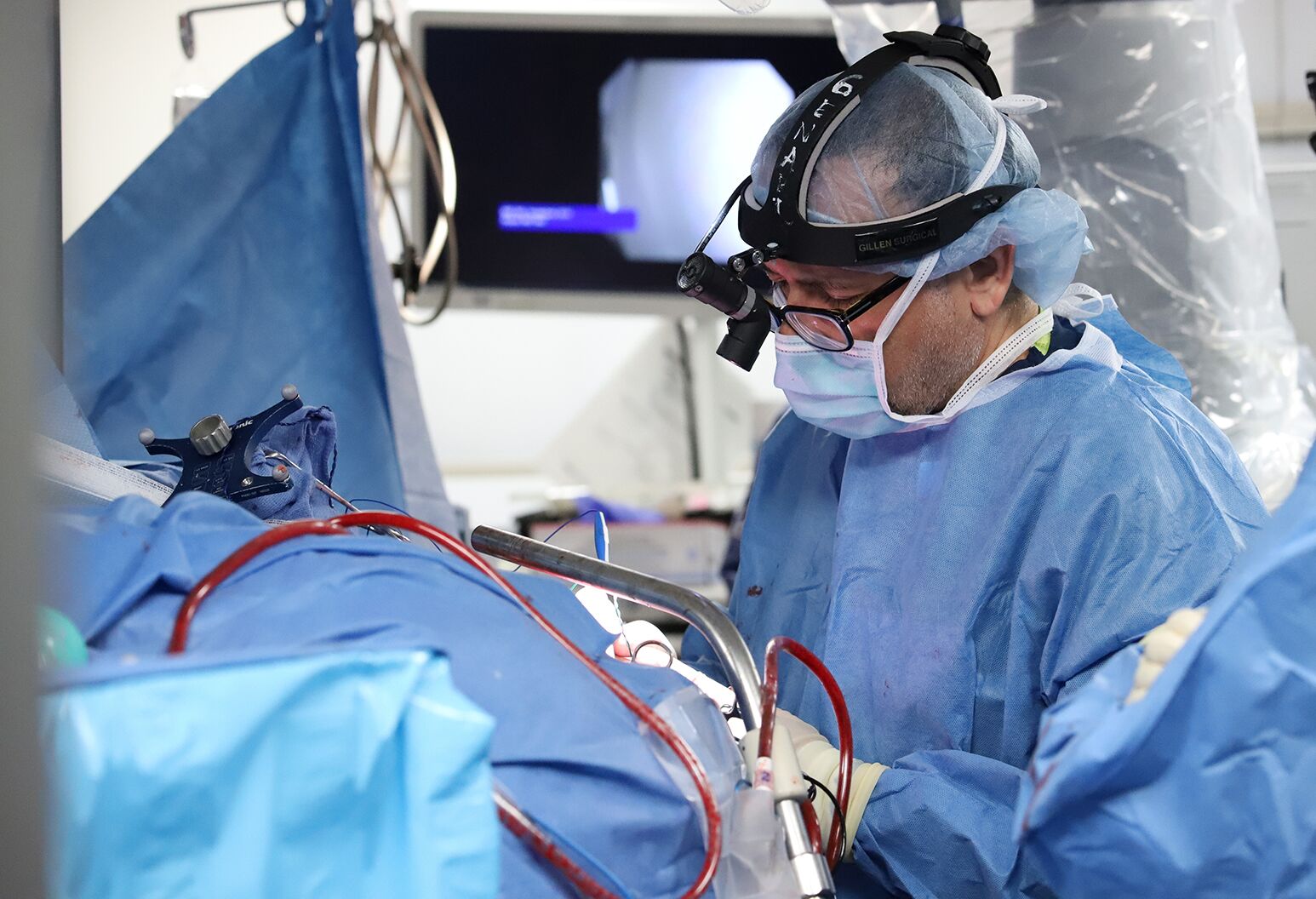
Ashesh Mehta, MD, implants five tiny computer chips into Keith's brain, key components of the novel ‘double neural bypass’ technology, which is helping restore not only movement, but also sensation in his arm and hand.
As Dr. Mehta stimulated his brain in the motor cortex, we were able to see muscle activity corresponding to neighboring areas not affected by the paralysis. We then moved on to confirm our targets in the sensory areas in Keith’s brain. As Dr. Mehta applied small amounts of electrical stimulation directly to the surface of his brain, he asked Keith whether he felt anything in his fingers — the intent was to evoke the sense of touch in his thumb, palm and index finger. Keith was able to report, in a faint whisper, that he felt sensation in all three — we selected these areas because of their role in many critical hand movements. He even mentioned feeling his pinky at one point.
During those 10 minutes he laid awake, Keith’s responses confirmed that the team’s preparations were paying off. The bioelectronic medicine research and clinical teams had accurately mapped and identified the areas of the brain crucial to interfacing the double neural bypass and restoring movement and the sense of touch — a moment that was years in the making.
With this critical step complete and our positioning confirmed, Dr. Mehta and his team seated the microchips on the surface of Keith’s brain. Finally, they secured two ports in the skull so that, even after surgery, we could connect electronically to these implants. In addition to helping us "decode" his electrical brain activity patterns to determine which movements he wants to make once again, the ports also allow us to apply stimulation to his brain. This stimulation prompts finger sensations and, when combined with spinal cord stimulation, may be the cause of the new sensations Keith is experiencing in his forearm and wrist — just months after surgery.
After Keith recovered from surgery, we resumed our weekly Tuesday, Thursday sessions, which took on new purpose. We had implanted a critical portion of the double neural bypass, the brain electrodes, in his motor and sensory areas, but needed to connect the remaining portions of the bypass in our laboratory to help restore his movement and sense of touch.
The double neural bypass owes its name to the two ‘branches’ that form the double bypass: the first is the brain-to-body branch, and second, is the brain-to-spinal cord branch. These two branches work together to help strengthen neural connections in Keith’s damaged spinal cord. The hope is that this will re-establish natural communication between his brain and paralyzed limbs over time.
The first branch or connection we formed was between the ports on Keith’s skull and a high-powered computer. The microelectrodes on the surface of his brain record electrical signals from his motor, and sometimes sensory, area and sends them to the computer for processing.
Over many years, we have developed AI algorithms to interpret and translate those electrical signals from his brain — like learning a new language, the AI uses that data to learn patterns and form associations, in this case, with arm and hand movements Keith would like to make. This allows us to, in effect, read his mind.
The double neural bypass can turn Keith’s intentions, like opening or closing his hand, or even moving individual fingers, into reality. The AI translates his brain activity patterns into intentions, and those intentions into action.
To close the loop, we then placed tiny sensors on his fingertips and palm, which send touch and pressure information back to the sensory area of his brain. Keith described it like something out of science fiction after the double neural bypass system allowed him to feel sensation in his fingertips once again.
The second branch of the double neural bypass involves reconnecting the brain to the spinal cord. Again, this process starts with Keith’s intentions. We asked him to think about squeezing his hand as if holding a bottle — a scenario represented on the computer screen. When he thinks about taking an action such as squeezing the bottle, electrical signals are sent from the brain implant to the computer, which then channels that information to flexible patches equipped with electrodes and placed over his spinal cord and around his forearm. These electrodes activate his muscles and can help restore movement. The patches stimulate the sensory roots of the spinal cord just below his injury and help reconnect and strengthen those damaged pathways.
While spinal cord stimulation has been possible for a number of years, this is the first time that the brain has been reconnected to the spinal cord to restore both movement and sensation. This allows the user — in this case Keith — to control the stimulation with their own thoughts, something we call ‘thought-driven therapy.’ Not only novel, this approach keeps the trial participant engaged and offers a sense of control.
This two-part signaling and stimulation completes our double neural bypass and, we hope, will continue to create long-lasting effects in both movement and touch.
One year after his double neural bypass surgery, Keith is making incredible progress at The Feinstein Institutes for Medical Research. Using only his thoughts, he can now grasp, lift and take a drink from a cup. Keith has regained even more sensation in his wrist and arm outside of the lab.
When we met Keith in October 2021, he couldn’t lift his arms off of his wheelchair and had no feeling below his mid-chest. Just one month after surgery, Keith reported sensation in the tips of his fingers when he uses the double neural bypass technology, and shortly after began to open his hand as well. Now, more than one year later, Keith has continued to experience lasting sensations in his forearms and wrist that are present even when we turn off the bypass, an early indication there may be natural recovery beginning to occur.
Spinal cord stimulation has in recent years shown some benefits in terms of awakening dormant circuits in the spinal cord and even strengthening and possibly rebuilding some of those connections. With the changes we are seeing in Keith and the large increase he continues to experience in arm mobility and strength, it is extremely encouraging that the double neural bypass technology may be a new treatment option in the future.
Working with other spinal cord injury patients, we recently published two studies showing that targeted spinal cord stimulation paired with minimal activity-based training resulted in a substantial and sustained increase in muscle activity and strength in specific upper-limb muscles.
Like any exercise, this takes time and repetition, and we believe Keith will continue to make gains. As Keith continues to make progress, his experience will lead us to new discoveries — and new hope for people with paralysis.
In the future, we think this technology could be used not only for spinal cord injury, but also for stroke recovery, and potentially for a wide range of other conditions including MS, Parkinson’s disease, and even traumatic brain injury.
Chad Bouton is professor and vice president of advanced engineering at the Feinstein Institutes for Medical Research at Northwell Health.
Our representatives are available to schedule your appointment Monday through Friday from 9am to 5pm.
For a Northwell ambulance, call
(833) 259-2367.